it is reported that in addition to being used in medicines and medical devices, sodium hyaluronate (sodium hyaluronate) products are also commonly used in cosmetics, food and other fields. some product uses are on the edge of medicines, medical devices and cosmetics.
11on march 14, the state food and drug administration issued the "announcement on the management categories of medical sodium hyaluronate products" (hereinafter referred to as the "announcement"). it is clear that sodium hyaluronate, as a moisturizing and hydrating ingredient, has been used in cosmetics. apply by rubbing, spraying or other similar methods toproducts containing sodium hyaluronate for the purpose of cleaning, protecting, modifying, and beautifying human body surfaces such as skin, hair, nails, lips, etc. are not regulated as drugs or medical devices.。such products should not make claims for medical use.
clarify the management attributes and management categories of related products
according to the "announcement", medical sodium hyaluronate products used to treat defects in the glucosamine protective layer of the bladder epithelium have been approved for marketing as class iii medical devices. this type of product is currently not marketed according to drug approval. in order to maintain the continuity of management, the original management attributes will continue to be maintained.
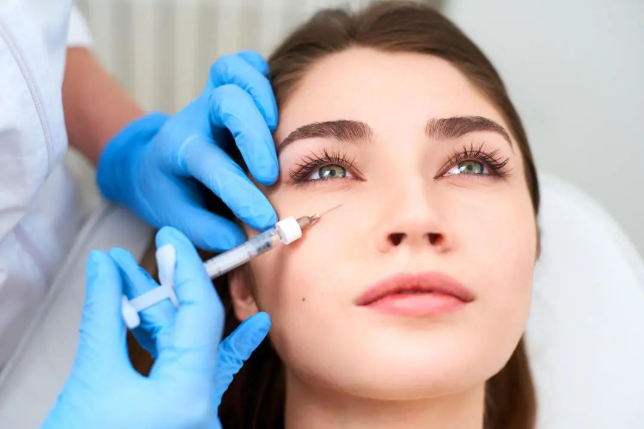
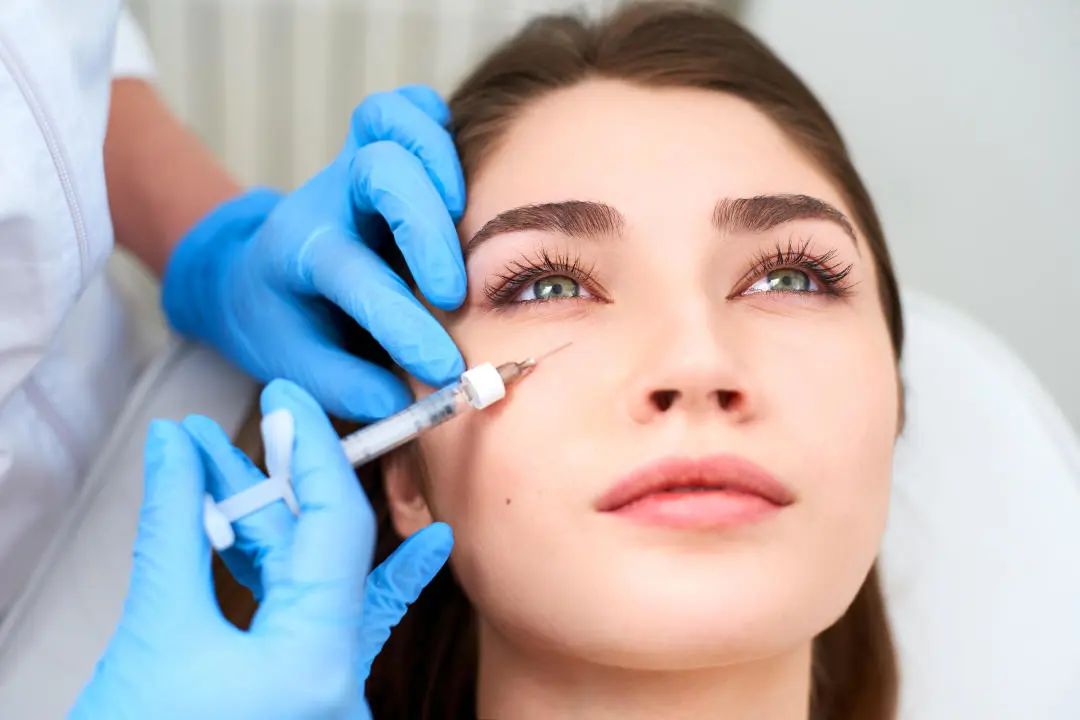
when medical sodium hyaluronate products are used for injection into the dermal layer of the skin and below, and are used as injection filling products to increase tissue volume, if the product does not contain pharmaceutical ingredients that exert pharmacological, metabolic or immunological effects, it shall be classified as class iii. medical device management; if the product contains local anesthetics and other drugs (such as lidocaine hydrochloride, amino acids, vitamins), it is determined to be a drug-device combination product based on medical devices.
when a medical sodium hyaluronate product is injected into the dermis to improve the skin condition mainly through the moisturizing and hydrating effects of the sodium hyaluronate it contains, if the product does not contain drugs that exert pharmacological, metabolic or immunological effects ingredients are managed according to the third category of medical devices; if the product contains local anesthetics and other drugs (such as lidocaine hydrochloride, amino acids, vitamins, etc.), it is determined to be a drug-device combination product based on medical devices.
medical dressings containing sodium hyaluronate, if they do not contain pharmaceutical ingredients that exert pharmacological, metabolic or immunological effects, are managed as medical devices; if they can be partially or fully absorbed by the human body or used on chronic wounds, they are managed as medical devices. category iii medical devices are managed; if they cannot be absorbed by the human body and are used on non-chronic wounds, they are managed as category ii medical devices.
in view of the scar repair materials that assist in improving pathological skin scars and assisting in preventing the formation of pathological skin scars, they have been included in the "medical device classification catalog" 14-12-02 scar repair materials and are managed as class ii medical devices. when such products contain sodium hyaluronate, their regulatory attributes and regulatory categories will not change.

the management category of medical sodium hyaluronate products is not lower than category 2
《"announcement" points out that sodium hyaluronate (sodium hyaluronate) is generally extracted from animal tissue or produced through microbial fermentation, and there are certain potential risks. the first-class medical device regulatory measures are difficult to guarantee its safety and effectiveness. therefore, according to the medical device management the management category of medical sodium hyaluronate (sodium hyaluronate) products should be no lower than category 2.
in addition, the "announcement" also clarifies that lotions, disinfectants, disinfectant pads, etc. containing disinfectant ingredients that are only used for disinfection of damaged skin and wounds are not managed as drugs or medical devices; modified sodium hyaluronate has been verified if the relevant physical, chemical, and biological properties are consistent with those of sodium hyaluronate, the management attributes and management categories can be implemented with reference to this announcement.